Acute myeloid leukemia (AML) is a disease of older patients with a median age at diagnosis of 68 years. Patients older than 65 years historically have performed poorly as not being treated with intensive chemotherapy. Biological age is not the only culprit as other factors also play a role in reducing survival, such as performance status, the presence of comorbid conditions, and most importantly the biology of AML that is usually associated with high risk cytogenetic or molecular aberrations. Despite the increasing use of targeted therapies, allogeneic hematopoietic cell transplantation (allo-HCT) remains an important and potentially curative treatment modality for patients with AML. Over time, significant progress in allo-HCT has resulted in decreased non-relapse mortality (NRM) and allowed the delivery of allo-HCT to older patients. However, little information is available about the global impact of the current standard of care for older AML patients after allo-HCT and about the predictive factors for post-transplant outcomes. To address these challenges, we assessed real-world changes over time in transplant characteristics and post-transplant outcomes in older patients with AML (> 65 years), using a large dataset from the European Society for Blood and Marrow Transplantation registry.
We identified 7215 adult patients (40% female; median age 68 years, range 65-80) with AML (secondary AML in 25%) allografted between 2000 and 2021 from a matched sibling donor (MSD; 23%), unrelated donor (UD; 63%) or haploidentical donor (Haplo; 14%). Karyotype risk was intermediate in 71% and adverse in 25%. NPM1 mutation and FLT3 ITD were present in 37% and 30% of patients, respectively.At transplant, 64% of patients were in first complete remission (CR1), 14% in CR2+ and 22% had active disease. Comorbidity index was zero in 43% of patients, 1-2 in 26% and >3 in 31%, while the Karnofsky score was <90 in 35%. Conditioning was reduced intensity (RIC) in 82% of patients. In vivo T cell depletion (TCD) and peripheral blood stem cells (PBSCs) were given to 62% and 93% of patients, respectively. Median follow up calculated by reverse Kaplan Meier was 40 months (interquartile range [IQR] 38-42).
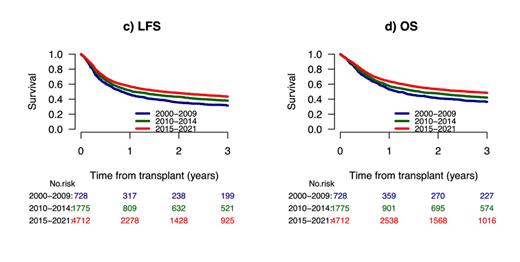
We compared changes in patient and transplant characteristics over time in 728 (10%) patients transplanted in 2000-2009, 1775 (25%) patients transplanted in 2010-2014, and 4712 (65%) patients transplanted in 2015-2021. Patients transplanted in recent years (65%) were older, were more likely to be in CR1 and to have an adverse-risk karyotype. Patients transplanted more recently were less likely to have had an MSD but more likely to have had a Haplo, PBSCs and TCD. The 3-year cumulative incidence of relapse (CIR) gradually and significantly decreased from 37% to 31%, then to 30% over the 3 time periods (p=0.001) whereas NRM significantly decreased from 31% and 31% to 27% (p=0.003). The 2-year leukemia free survival (LFS) and overall survival (OS) gradually and significantly improved over time from 32% to 38%, and then to 44% (p=0.001) and from 37% to 42%, and then to 49% (p=0.001), respectively, whereas graft-versus-host disease (GVHD)-free, relapse-free survival (GRFS) improved from 22% to 29%, and then to 34% (p=0.001). In a Cox regression multivariate analysis (MVA), a significant reduction in CIR and increased LFS, OS and GRFS were noted after 2015. LFS, OS and GRFS were also negatively affected by secondary AML, low Karnofsky score, active disease at transplant, and adverse-risk cytogenetics. GRFS was also negatively affected by older age, and positively affected by TCD. RIC positively affected OS whereas PBSCs positively affected LFS and OS. This improvement of post-transplant outcomes over time was observed for patients in CR1 with LFS and OS increasing from 39% to 44% and then to 47% (p=0.005) and from 43% to 48%, and then to 52% (p=0.006) as well as for patients in CR2 with LFS and OS increasing from 29% to 40% and then to 47% (p=0.001) and from 37% to 44% and then to 52% (p= 0.001), respectively.
In conclusion, in older patients with AML, we observed an impressive improvement over time in post-transplant outcomes with decreased CIR and improved LFS, OS and GRFS. These large-scale, real-world data can serve as a benchmark for future studies in this setting and indicate that the opportunity for transplant for the elderly should be mandatory and no longer an option.
Disclosures
Schetelig:Eurocept: Honoraria; BeiGene: Consultancy, Honoraria; Novartis: Honoraria; Abbvie: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; BMS: Consultancy, Honoraria. Finke:Gilead Sciences: Current holder of stock options in a privately-held company; AbbVie: Current holder of stock options in a privately-held company; Roche: Current holder of stock options in a privately-held company; Riemser: Honoraria, Research Funding, Speakers Bureau; Neovii: Honoraria, Research Funding, Speakers Bureau; Medac: Honoraria, Research Funding. Platzbecker:Novartis: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Research Funding; Curis: Consultancy, Research Funding; AbbVie: Consultancy; BMS: Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support; medical writing support, Research Funding; Janssen Biotech: Consultancy, Research Funding; Silence Therapeutics: Consultancy, Honoraria, Research Funding; Celgene: Honoraria; MDS Foundation: Membership on an entity's Board of Directors or advisory committees; Roche: Research Funding; Fibrogen: Research Funding; Geron: Consultancy, Research Funding; Merck: Research Funding; Jazz: Consultancy, Honoraria, Research Funding; BeiGene: Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Syros: Consultancy, Honoraria, Research Funding; Servier: Consultancy, Honoraria, Research Funding. Dreger:Novartis: Consultancy, Speakers Bureau; BMS: Consultancy, Honoraria; Gilead: Consultancy, Speakers Bureau; Riemser: Consultancy, Research Funding, Speakers Bureau; Beigene: Consultancy, Honoraria; AstraZeneca: Consultancy, Speakers Bureau; Abbvie: Consultancy, Speakers Bureau; Roche: Consultancy, Speakers Bureau; Miltenyi: Consultancy. Ciceri:ExCellThera: Other: Scientific Advisory Board . Mohty:JAZZ PHARMACEUTICALS: Honoraria, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal